Corvert: Package Insert / Prescribing Info
Package insert / product label
Generic name: ibutilide fumarate
Dosage form: injection, solution
Drug class: Group III antiarrhythmics
J Code (medical billing code): J1742 (Per 1 mg, injection)
Medically reviewed by Drugs.com. Last updated on Jan 31, 2024.
On This Page
LIFE-THREATENING ARRHYTHMIAS—APPROPRIATE TREATMENT ENVIRONMENT
CORVERT can cause potentially fatal arrhythmias, particularly sustained polymorphic ventricular tachycardia, usually in association with QT prolongation (torsades de pointes), but sometimes without documented QT prolongation. In registration studies, these arrhythmias, which require cardioversion, occurred in 1.7% of treated patients during, or within a number of hours of, use of CORVERT. These arrhythmias can be reversed if treated promptly (see WARNINGS, Proarrhythmia). It is essential that CORVERT be administered in a setting of continuous ECG monitoring and by personnel trained in identification and treatment of acute ventricular arrhythmias, particularly polymorphic ventricular tachycardia. Patients with atrial fibrillation of more than 2 to 3 days' duration must be adequately anticoagulated, generally for at least 2 weeks.
CHOICE OF PATIENTS
Patients with chronic atrial fibrillation have a strong tendency to revert after conversion to sinus rhythm (see CLINICAL STUDIES) and treatments to maintain sinus rhythm carry risks. Patients to be treated with CORVERT, therefore, should be carefully selected such that the expected benefits of maintaining sinus rhythm outweigh the immediate risks of CORVERT, and the risks of maintenance therapy, and are likely to offer an advantage compared with alternative management.
Corvert Description
CORVERT Injection (ibutilide fumarate injection) is an antiarrhythmic drug with predominantly class III (cardiac action potential prolongation) properties according to the Vaughan Williams Classification. Each milliliter of CORVERT Injection contains 0.1 mg of ibutilide fumarate (equivalent to 0.087 mg ibutilide free base), 0.189 mg sodium acetate trihydrate, 8.90 mg sodium chloride, hydrochloric acid to adjust pH to approximately 4.6, and Water for Injection.
CORVERT Injection is an isotonic, clear, colorless, sterile aqueous solution.
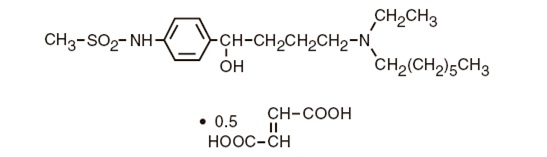
Ibutilide fumarate has one chiral center, and exists as a racemate of the (+) and (−) enantiomers.
The chemical name for ibutilide fumarate is Methanesulfonamide, N-{4-{4-(ethylheptylamino)-1-hydroxybutyl}phenyl}, (+) (−), (E)-2-butenedioate (1:0.5) (hemifumarate salt). Its molecular formula is C22H38N2O5S, and its molecular weight is 442.62.
Ibutilide fumarate is a white to off-white powder with an aqueous solubility of over 100 mg/mL at pH 7 or lower.
The structural formula of ibutilide fumarate is represented below:
Corvert - Clinical Pharmacology
Mechanism of Action:
CORVERT Injection prolongs action potential duration in isolated adult cardiac myocytes and increases both atrial and ventricular refractoriness in vivo, ie, class III electrophysiologic effects. Voltage clamp studies indicate that CORVERT, at nanomolar concentrations, delays repolarization by activation of a slow, inward current (predominantly sodium), rather than by blocking outward potassium currents, which is the mechanism by which most other class III antiarrhythmics act. These effects lead to prolongation of atrial and ventricular action potential duration and refractoriness, the predominant electrophysiologic properties of CORVERT in humans that are thought to be the basis for its antiarrhythmic effect.
Electrophysiologic Effects:
CORVERT produces mild slowing of the sinus rate and atrioventricular conduction. CORVERT produces no clinically significant effect on QRS duration at intravenous doses up to 0.03 mg/kg administered over a 10-minute period. Although there is no established relationship between plasma concentration and antiarrhythmic effect, CORVERT produces dose-related prolongation of the QT interval, which is thought to be associated with its antiarrhythmic activity. (See WARNINGS for relationship between QTc prolongation and torsades de pointes-type arrhythmias.) In a study in healthy volunteers, intravenous infusions of CORVERT resulted in prolongation of the QT interval that was directly correlated with ibutilide plasma concentration during and after 10-minute and 8-hour infusions. A steep ibutilide concentration/response (QT prolongation) relationship was shown. The maximum effect was a function of both the dose of CORVERT and the infusion rate.
Hemodynamic Effects:
A study of hemodynamic function in patients with ejection fractions both above and below 35% showed no clinically significant effects on cardiac output, mean pulmonary arterial pressure, or pulmonary capillary wedge pressure at doses of CORVERT up to 0.03 mg/kg.
Pharmacokinetics:
After intravenous infusion, ibutilide plasma concentrations rapidly decrease in a multiexponential fashion. The pharmacokinetics of ibutilide are highly variable among subjects. Ibutilide has a high systemic plasma clearance that approximates liver blood flow (about 29 mL/min/kg), a large steady-state volume of distribution (about 11 L/kg) in healthy volunteers, and minimal (about 40%) protein binding. Ibutilide is also cleared rapidly and highly distributed in patients being treated for atrial flutter or atrial fibrillation. The elimination half-life averages about 6 hours (range from 2 to 12 hours). The pharmacokinetics of ibutilide are linear with respect to the dose of CORVERT over the dose range of 0.01 mg/kg to 0.10 mg/kg. The enantiomers of ibutilide fumarate have pharmacokinetic properties similar to each other and to ibutilide fumarate.
The pharmacokinetics of CORVERT Injection in patients with atrial flutter or atrial fibrillation are similar regardless of the type of arrhythmia, patient age, sex, or the concomitant use of digoxin, calcium channel blockers, or beta blockers.
Metabolism and elimination:
In healthy male volunteers, about 82% of a 0.01 mg/kg dose of [14C] ibutilide fumarate was excreted in the urine (about 7% of the dose as unchanged ibutilide) and the remainder (about 19%) was recovered in the feces.
Eight metabolites of ibutilide were detected in metabolic profiling of urine. These metabolites are thought to be formed primarily by ω-oxidation followed by sequential β-oxidation of the heptyl side chain of ibutilide. Of the eight metabolites, only the ω-hydroxy metabolite possesses class III electrophysiologic properties similar to that of ibutilide in an in vitro isolated rabbit myocardium model. The plasma concentrations of this active metabolite, however, are less than 10% of that of ibutilide.
Clinical Studies:
Treatment with intravenous ibutilide fumarate for acute termination of recent onset atrial flutter/fibrillation was evaluated in 466 patients participating in two randomized, double-blind, placebo-controlled clinical trials. Patients had had their arrhythmias for 3 hours to 90 days, were anticoagulated for at least 2 weeks if atrial fibrillation was present more than 3 days, had serum potassium of at least 4.0 mEq/L and QTc below 440 msec, and were monitored by telemetry for at least 24 hours. Patients could not be on class I or other class III antiarrhythmics (these had to be discontinued at least 5 half-lives prior to infusion) but could be on calcium channel blockers, beta blockers, or digoxin. In one trial, single 10-minute infusions of 0.005 to 0.025 mg/kg were tested in parallel groups (0.3 to 1.5 mg in a 60 kg person). In the second trial, up to two infusions of ibutilide fumarate were evaluated—the first 1.0 mg, the second given 10 minutes after completion of the first infusion, either 0.5 or 1.0 mg. In a third double-blind study, 319 patients with atrial fibrillation or atrial flutter of 3 hours to 45 days duration were randomized to receive single, 10-minute intravenous infusions of either sotalol (1.5 mg/kg) or CORVERT (1 mg or 2 mg). Among patients with atrial flutter, 53% receiving 1 mg ibutilide fumarate and 70% receiving 2 mg ibutilide fumarate converted, compared to 18% of those receiving sotalol. In patients with atrial fibrillation, 22% receiving 1 mg ibutilide fumarate and 43% receiving 2 mg ibutilide fumarate converted compared to 10% of patients receiving sotalol.
Patients in registration trials were hemodynamically stable. Patients with specific cardiovascular conditions such as symptomatic heart failure, recent acute myocardial infarction, and angina were excluded. About two thirds had cardiovascular symptoms, and the majority of patients had left atrial enlargement, decreased left ventricular ejection fraction, a history of valvular disease, or previous history of atrial fibrillation or flutter. Electrical cardioversion was allowed 90 minutes after the infusion was complete. Patients could be given other antiarrhythmic drugs 4 hours postinfusion.
Results of the first two studies are shown in the tables below. Conversion of atrial flutter/fibrillation usually (70% of those who converted) occurred within 30 minutes of the start of infusion and was dose related. The latest conversion seen was at 90 minutes after the start of the infusion. Most converted patients remained in normal sinus rhythm for 24 hours. Overall responses in these patients, defined as termination of arrhythmias for any length of time during or within 1 hour following completed infusion of randomized dose, were in the range of 43% to 48% at doses above 0.0125 mg/kg (vs 2% for placebo). Twenty-four hour responses were similar. For these atrial arrhythmias, ibutilide was more effective in patients with flutter than fibrillation ( ≥ 48% vs ≤ 40%).
| PERCENT OF PATIENTS WHO CONVERTED (First Trial) | ||||||
|---|---|---|---|---|---|---|
| Ibutilide | ||||||
| Placebo | 0.005 mg/kg | 0.01 mg/kg | 0.015 mg/kg | 0.025 mg/kg | ||
| n | 41 | 41 | 40 | 38 | 40 | |
|
Both |
Initially* |
2 |
12 |
33 |
45 |
48 |
|
At 24 hours† |
2 |
12 |
28 |
42 |
43 |
|
|
Atrial flutter |
Initially* |
0 |
14 |
30 |
58 |
55 |
|
At 24 hours† |
0 |
14 |
30 |
58 |
50 |
|
|
Atrial fibrillation |
Initially* |
5 |
10 |
35 |
32 |
40 |
|
At 24 hours† |
5 |
10 |
25 |
26 |
35 |
|
| PERCENT OF PATIENTS WHO CONVERTED (Second Trial) | ||||
|---|---|---|---|---|
| Ibutilide | ||||
| Placebo | 1.0 mg/0.5 mg | 1.0 mg/1.0 mg | ||
| n | 86 | 86 | 94 | |
|
Both |
Initially* |
2 |
43 |
44 |
|
At 24 hours† |
2 |
34 |
37 |
|
|
Atrial flutter |
Initially* |
2 |
48 |
63 |
|
At 24 hours† |
2 |
45 |
59 |
|
|
Atrial fibrillation |
Initially* |
2 |
38 |
25 |
|
At 24 hours† |
2 |
21 |
17 |
|
The numbers of patients who remained in the converted rhythm at the end of 24 hours were slightly less than those patients who converted initially, but the difference between conversion rates for ibutilide compared to placebo was still statistically significant. In long-term follow-up, approximately 40% of all patients remained recurrence free, usually with chronic prophylactic treatment, 400 to 500 days after acute treatment, regardless of the method of conversion.
Patients with more recent onset of arrhythmia had a higher rate of conversion. Response rates were 42% and 50% for patients with onset of atrial fibrillation/flutter for less than 30 days in the two efficacy studies compared to 16% and 31% in those with more chronic arrhythmias.
Ibutilide was equally effective in patients below and above 65 years of age and in men and women. Female patients constituted about 20% of patients in controlled studies.
Post-cardiac Surgery:
In a double-blind, parallel group study, 302 patients with atrial fibrillation (n=201) or atrial flutter (n=101) that occurred 1 to 7 days after coronary artery bypass graft or valvular surgery and lasted 1 hour to 3 days were randomized to receive two 10-minute infusions of placebo, or 0.25, 0.5 or 1 mg of ibutilide fumarate. Among patients with atrial flutter, conversion rates at 1.5 hours were: placebo, 4%; 0.25 mg ibutilide fumarate, 56%; 0.5 mg ibutilide fumarate, 61%; and 1 mg ibutilide fumarate, 78%. Among patients with atrial fibrillation, conversion rates at 1.5 hours were: placebo, 20%; 0.25 mg ibutilide fumarate, 28%; 0.5 mg ibutilide fumarate, 42%, and 1 mg ibutilide fumarate, 44%. The majority of patients (53% and 72% in the 0.5-mg and 1-mg dose groups, respectively) converted to sinus rhythm remained in sinus rhythm for 24 hours. Patients were not given other antiarrhythmic drugs within 24 hours of ibutilide fumarate infusion in this study.
Indications and Usage for Corvert
CORVERT Injection is indicated for the rapid conversion of atrial fibrillation or atrial flutter of recent onset to sinus rhythm. Patients with atrial arrhythmias of longer duration are less likely to respond to CORVERT. The effectiveness of ibutilide has not been determined in patients with arrhythmias of more than 90 days in duration.
Contraindications
CORVERT Injection is contraindicated in patients who have previously demonstrated hypersensitivity to ibutilide fumarate or any of the other product components.
Warnings
Proarrhythmia:
Like other antiarrhythmic agents, CORVERT Injection can induce or worsen ventricular arrhythmias in some patients. This may have potentially fatal consequences. Torsades de pointes, a polymorphic ventricular tachycardia that develops in the setting of a prolonged QT interval, may occur because of the effect CORVERT has on cardiac repolarization, but CORVERT can also cause polymorphic VT in the absence of excessive prolongation of the QT interval. In general, with drugs that prolong the QT interval, the risk of torsades de pointes is thought to increase progressively as the QT interval is prolonged and may be worsened with bradycardia, a varying heart rate, and hypokalemia. In clinical trials conducted in patients with atrial fibrillation and atrial flutter, those with QTc intervals >440 msec were not usually allowed to participate, and serum potassium had to be above 4.0 mEq/L. Although change in QTc was dose dependent for ibutilide, there was no clear relationship between risk of serious proarrhythmia and dose in clinical studies, possibly due to the small number of events. In clinical trials of intravenous ibutilide, patients with a history of congestive heart failure (CHF) or low left ventricular ejection fraction appeared to have a higher incidence of sustained polymorphic ventricular tachycardia (VT), than those without such underlying conditions; for sustained polymorphic VT the rate was 5.4% in patients with a history of CHF and 0.8% without it. There was also a suggestion that women had a higher risk of proarrhythmia, but the sex difference was not observed in all studies and was most prominent for nonsustained ventricular tachycardia. The incidence of sustained ventricular arrhythmias was similar in male (1.8%) and female (1.5%) patients, possibly due to the small number of events. CORVERT is not recommended in patients who have previously demonstrated polymorphic ventricular tachycardia (eg, torsades de pointes).
During registration trials, 1.7% of patients with atrial flutter or atrial fibrillation treated with CORVERT developed sustained polymorphic ventricular tachycardia requiring cardioversion. In these clinical trials, many initial episodes of polymorphic ventricular tachycardia occurred after the infusion of CORVERT was stopped but generally not more than 40 minutes after the start of the first infusion. There were, however, instances of recurrent polymorphic VT that occurred about 3 hours after the initial infusion. In two cases, the VT degenerated into ventricular fibrillation, requiring immediate defibrillation. Other cases were managed with cardiac pacing and magnesium sulfate infusions. Nonsustained polymorphic ventricular tachycardia occurred in 2.7% of patients and nonsustained monomorphic ventricular tachycardias occurred in 4.9% of the patients (see ADVERSE REACTIONS).
Proarrhythmic events must be anticipated. Skilled personnel and proper equipment, including cardiac monitoring equipment, intracardiac pacing facilities, a cardioverter/defibrillator, and medication for treatment of sustained ventricular tachycardia, including polymorphic ventricular tachycardia, must be available during and after administration of CORVERT. Before treatment with CORVERT, hypokalemia and hypomagnesemia should be corrected to reduce the potential for proarrhythmia. Patients should be observed with continuous ECG monitoring for at least 4 hours following infusion or until QTc has returned to baseline. Longer monitoring is required if any arrhythmic activity is noted. Management of polymorphic ventricular tachycardia includes discontinuation of ibutilide, correction of electrolyte abnormalities, especially potassium and magnesium, and overdrive cardiac pacing, electrical cardioversion, or defibrillation. Pharmacologic therapies include magnesium sulfate infusions. Treatment with antiarrhythmics should generally be avoided.
Precautions
General
Antiarrhythmics:
Class Ia antiarrhythmic drugs (Vaughan Williams Classification), such as disopyramide, quinidine, and procainamide, and other class III drugs, such as amiodarone and sotalol, should not be given concomitantly with CORVERT Injection or within 4 hours postinfusion because of their potential to prolong refractoriness. In the clinical trials, class I or other class III antiarrhythmic agents were withheld for at least 5 half-lives prior to ibutilide infusion and for 4 hours after dosing, but thereafter were allowed at the physician's discretion.
Other drugs that prolong the QT interval:
The potential for proarrhythmia may increase with the administration of CORVERT Injection to patients who are being treated with drugs that prolong the QT interval, such as phenothiazines, tricyclic antidepressants, tetracyclic antidepressants, and certain antihistamine drugs (H1 receptor antagonists).
Drug Interactions:
No specific pharmacokinetic or other formal drug interaction studies were conducted.
Digoxin:
Supraventricular arrhythmias may mask the cardiotoxicity associated with excessive digoxin levels. Therefore, it is advisable to be particularly cautious in patients whose plasma digoxin levels are above or suspected to be above the usual therapeutic range. Coadministration of digoxin did not have effects on either the safety or efficacy of ibutilide in the clinical trials.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
No animal studies have been conducted to determine the carcinogenic potential of CORVERT; however, it was not genotoxic in a battery of assays, (Ames assay, mammalian cell forward gene mutation assay, unscheduled DNA synthesis assay, and mouse micronucleus assay). Similarly, no drug-related effects on fertility or mating were noted in a reproductive study in rats in which ibutilide was administered orally to both sexes up to doses of 20 mg/kg/day. On a mg/m2 basis, corrected for 3% bioavailability, the highest dose tested was approximately four times the maximum recommended human dose (MRHD).
Pregnancy:
Ibutilide administered orally was teratogenic (abnormalities included adactyly, interventricular septal defects, and scoliosis) and embryocidal in reproduction studies in rats. On a mg/m2 basis, corrected for the 3% oral bioavailability, the "no adverse effect dose" (5 mg/kg/day given orally) was approximately the same as the maximum recommended human dose (MRHD); the teratogenic dose (20 mg/kg/day given orally) was about four times the MRHD on a mg/m2 basis, or 16 times the MRHD on a mg/kg basis. CORVERT should not be administered to a pregnant woman unless clinical benefit outweighs potential risk to the fetus.
Nursing Mothers:
The excretion of ibutilide into breast milk has not been studied; accordingly, breastfeeding should be discouraged during therapy with CORVERT.
Pediatric Use:
Clinical trials with CORVERT in patients with atrial fibrillation and atrial flutter did not include anyone under the age of 18. Safety and effectiveness of ibutilide in pediatric patients has not been established.
Geriatric Use:
Clinical studies of ibutilide fumarate (involving 586 patients) did not include sufficient numbers of subjects less than age 65 (45%) to determine whether they respond differently from older subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Use in Patients With Hepatic or Renal Dysfunction:
The safety, effectiveness, and pharmacokinetics of CORVERT have not been established in patients with hepatic or renal dysfunction. However, it is unlikely that dosing adjustments would be necessary in patients with compromised renal or hepatic function based on the following considerations: (1) CORVERT is indicated for rapid intravenous therapy (duration ≤ 30 minutes) and is dosed to a known, well-defined pharmacologic action (termination of arrhythmia) or to a maximum of two 10-minute infusions; (2) less than 10% of the dose of CORVERT is excreted unchanged in the urine; and (3) drug distribution appears to be one of the primary mechanisms responsible for termination of the pharmacologic effect. Nonetheless, patients with abnormal liver function should be monitored by telemetry for more than the 4-hour period generally recommended.
In 285 patients with atrial fibrillation or atrial flutter who were treated with CORVERT, the clearance of ibutilide was independent of renal function, as assessed by creatinine clearance (range 21 to 140 mL/min).
Adverse Reactions/Side Effects
CORVERT Injection was generally well tolerated in clinical trials. Of the 586 patients with atrial fibrillation or atrial flutter who received CORVERT in phase II/III studies, 149 (25%) reported medical events related to the cardiovascular system, including sustained polymorphic ventricular tachycardia (1.7%) and nonsustained polymorphic ventricular tachycardia (2.7%).
Other clinically important adverse events with an uncertain relationship to CORVERT include the following (0.2% represents one patient): sustained monomorphic ventricular tachycardia (0.2%), nonsustained monomorphic ventricular tachycardia (4.9%), AV block (1.5%), bundle branch block (1.9%), ventricular extrasystoles (5.1%), supraventricular extrasystoles (0.9%), hypotension/postural hypotension (2.0%), bradycardia/sinus bradycardia (1.2%), nodal arrhythmia (0.7%), congestive heart failure (0.5%), tachycardia/sinus tachycardia/supraventricular tachycardia (2.7%), idioventricular rhythm (0.2%), syncope (0.3%), and renal failure (0.3%). The incidence of these events, except for syncope, was greater in the group treated with CORVERT than in the placebo group.
Another adverse reaction that may be associated with the administration of CORVERT was nausea, which occurred with a frequency greater than 1% more in ibutilide-treated patients than those treated with placebo.
The medical events reported for more than 1% of the placebo- and ibutilide-treated patients are shown in the following Table.
| Event | Placebo N=127 | All Ibutilide N=586 |
||
|---|---|---|---|---|
| Patients | Patients | |||
| n | % | n | % | |
|
CARDIOVASCULAR | ||||
|
Ventricular extrasystoles |
1 |
0.8 |
30 |
5.1 |
|
Nonsustained monomorphic VT |
1 |
0.8 |
29 |
4.9 |
|
Nonsustained polymorphic VT |
— |
— |
16 |
2.7 |
|
Hypotension |
2 |
1.6 |
12 |
2.0 |
|
Bundle branch block |
— |
— |
11 |
1.9 |
|
Sustained polymorphic VT |
— |
— |
10 |
1.7 |
|
AV block |
1 |
0.8 |
9 |
1.5 |
|
Hypertension |
— |
— |
7 |
1.2 |
|
QT segment prolonged |
— |
— |
7 |
1.2 |
|
Bradycardia |
1 |
0.8 |
7 |
1.2 |
|
Palpitation |
1 |
0.8 |
6 |
1.0 |
|
Tachycardia |
1 |
0.8 |
16 |
2.7 |
|
GASTROINTESTINAL | ||||
|
Nausea |
1 |
0.8 |
11 |
1.9 |
|
CENTRAL NERVOUS SYSTEM | ||||
|
Headache |
4 |
3.1 |
21 |
3.6 |
In the post-cardiac surgery study (see CLINICAL STUDIES), similar types of medical events were reported. In the 1 mg ibutilide fumarate treatment group (N=70), 2 patients (2.9%) developed sustained polymorphic ventricular tachycardia and 2 other patients (2.9%) developed nonsustained polymorphic ventricular tachycardia. Polymorphic ventricular tachycardia was not reported in the 73 patients in the 0.5 mg dose group or in the 75 patients in the 0.25 mg dose group.
Overdosage
Acute Experience in Animals:
Acute overdose in animals results in CNS toxicity; notably, CNS depression, rapid gasping breathing, and convulsions. The intravenous median lethal dose in the rat was more than 50 mg/kg which is, on a mg/m2 basis, at least 250 times the maximum recommended human dose.
Human Experience:
In the registration trials with CORVERT Injection, four patients were unintentionally overdosed. The largest dose was 3.4 mg administered over 15 minutes. One patient (0.025 mg/kg) developed increased ventricular ectopy and monomorphic ventricular tachycardia, another patient (0.032 mg/kg) developed AV block—3rd degree and nonsustained polymorphic VT, and two patients (0.038 and 0.020 mg/kg) had no medical event reports. Based on known pharmacology, the clinical effects of an overdosage with ibutilide could exaggerate the expected prolongation of repolarization seen at usual clinical doses. Medical events (eg, proarrhythmia, AV block) that occur after the overdosage should be treated with measures appropriate for that condition.
Corvert Dosage and Administration
The recommended dose based on controlled trials (see CLINICAL STUDIES) is outlined in the Table below. Ibutilide infusion should be stopped as soon as the presenting arrhythmia is terminated or in the event of sustained or nonsustained ventricular tachycardia, or marked prolongation of QT or QTc.
| Patient Weight | Initial Infusion (over 10 minutes) | Second Infusion |
|---|---|---|
|
60 kg (132 lb)
|
One vial
|
If the arrhythmia does not terminate within 10 minutes after the end of the initial infusion, a second 10-minute infusion of equal strength may be administered 10 minutes after completion of the first infusion. |
|
Less than 60 kg
|
0.1 mL/kg
|
In a trial comparing ibutilide and sotalol (see CLINICAL STUDIES), 2 mg ibutilide fumarate administered as a single infusion to patients weighing more than 60 kg was also effective in terminating atrial fibrillation or atrial flutter.
In the post-cardiac surgery study (see CLINICAL STUDIES), one or two intravenous infusions of 0.5 mg (0.005 mg/kg per dose for patients weighing less than 60 kg) was effective in terminating atrial fibrillation or atrial flutter.
Patients should be observed with continuous ECG monitoring for at least 4 hours following infusion or until QTc has returned to baseline. Longer monitoring is required if any arrhythmic activity is noted. Skilled personnel and proper equipment (see WARNINGS, Proarrhythmia), such as a cardioverter/defibrillator, and medication for treatment of sustained ventricular tachycardia, including polymorphic ventricular tachycardia, must be available during administration of CORVERT and subsequent monitoring of the patient.
Dilution:
CORVERT Injection may be administered undiluted or diluted in 50 mL of diluent. CORVERT may be added to 0.9% Sodium Chloride Injection or 5% Dextrose Injection before infusion. The contents of one 10 mL vial (0.1 mg/mL) may be added to a 50 mL infusion bag to form an admixture of approximately 0.017 mg/mL ibutilide fumarate. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Compatibility and Stability:
The following diluents are compatible with CORVERT Injection (0.1 mg/mL):
- 5% Dextrose Injection
- 0.9% Sodium Chloride Injection
The following intravenous solution containers are compatible with admixtures of CORVERT Injection (0.1 mg/mL):
- polyvinyl chloride plastic bags
- polyolefin bags
Admixtures of the product, with approved diluents, are chemically and physically stable for 24 hours at room temperature (15° to 30° C or 59° to 86° F) and for 48 hours at refrigerated temperatures (2° to 8°C or 36° to 46°F). Strict adherence to the use of aseptic technique during the preparation of the admixture is recommended in order to maintain sterility.
How is Corvert supplied
CORVERT Injection (ibutilide fumarate injection) is supplied as an acetate-buffered isotonic solution at a concentration of 0.1 mg/mL that has been adjusted to approximately pH 4.6 in 10 mL clear glass, single-dose, flip-top vials.
Single-dose 10 mL vial, 1 mg /10 mL (0.1 mg/mL) NDC 0009-3794-22
Distributed by
Pharmacia & Upjohn Co
Division of Pfizer Inc, NY, NY 10001
Novaplus is a registered trademark of Vizient, Inc.
LAB-0385-5.0
November 2023
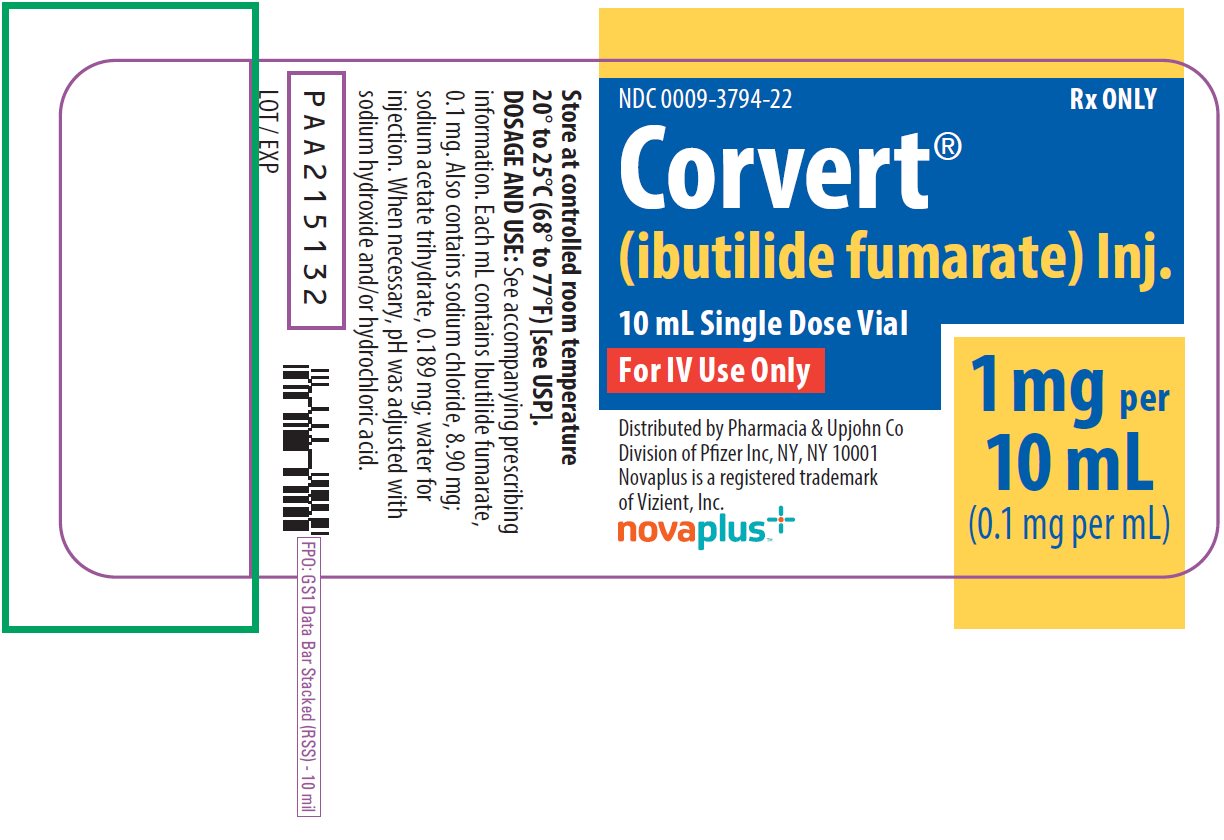
PRINCIPAL DISPLAY PANEL - 10 mL Vial Label
NDC 0009-3794-22
Rx ONLY
Corvert®
(ibutilide fumarate) Inj.
10 mL Single Dose Vial
For IV Use Only
1 mg per
10 mL
(0.1 mg per mL)
Distributed by Pharmacia & Upjohn Co
Division of Pfizer Inc, NY, NY 10001
Novaplus is a registered trademark
of Vizient, Inc.
novaplus™
| CORVERT
ibutilide fumarate injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pharmacia & Upjohn Company LLC (618054084) |
| Registrant - Pfizer Inc (113480771) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia & Upjohn Company LLC | 618054084 | ANALYSIS(0009-3794) , MANUFACTURE(0009-3794) , API MANUFACTURE(0009-3794) , PACK(0009-3794) , LABEL(0009-3794) | |
More about Corvert (ibutilide)
- Check interactions
- Compare alternatives
- Pricing & coupons
- Side effects
- Dosage information
- During pregnancy
- Generic availability
- Drug class: group III antiarrhythmics
- En español